|
Fullerene-like structures |
|||
|
C60
molecule pattern of a soccer ball |
|
Montreal Expo67 |
|
|
Dome designs by Buckminster Fuller |
|||
|
Fullerenes are a
new family of carbon besides diamond and graphite. In fullerenes the planar
structure of graphite is converted into quasi-icosahedral cages by 12
pentagonal "defects". Such an extremely stable cage is the C60
molecule, which copies the pattern of a soccer ball. Researchers have been
looking for ways to use the soccer-ball-shaped molecules of pure carbon known
as fullerenes (or buckyballs) since they were first synthesized, more than a
decade ago. But the solid materials they form are often weak and easily
degraded because the individual spheres hold to their neighbors through weak
van der Waals forces, rather than forming sturdy bonds. Addition of nitrogen
into the graphitic sheets makes it favourable to form pentagons and induce
curvature of the sheets. Therefore the fullerene structure can be synthesised
at lower temperatures. The fullerene-like shells can grow in an encapsulated
manner forming so called nano-onions. The incorporated nitrogen also helps to
strengthen the material by allowing the spheres to form tight covalent bonds
between each other. The onions were identified by TEM and analyzed by EELS.
Each "onion" was a few nanometers across and contained a core of C48N12
a new aza-fullerene. The onions seem to be strongly bonded to each other,
based on structural calculations and mechanical tests. The mechanical
properties were characterized by nanoindentation using a sharp diamond-tipped
probe. According to the mechanical tests, the material is fracture tough due
to its extreme elasticity making the material good for protective coatings on
hard disks, bearings and medical implants. The material holds the potential
in other applications as well, such as solid lubricants or electron emitters
in flat panel displays. The short range order of different
carbon based nanostructures can be modeled by simulation of the electron
diffraction of crystalline or polyhedral nanoclustres. |
|||
|
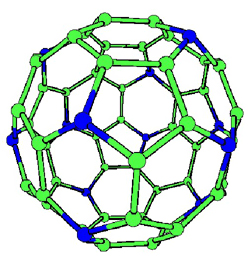
CNx nano-onions |
C48N12
aza-fullerene When 12 carbon atoms of aC60 molecule are replaced by nitrogens, it can link up with other "buckyballs" to form a strong and springy material. |
||